Rekom Biotech
Quality
Rekom Biotech is committed to ensuring the highest level of quality in the design and manufacture of our IVD reagents.
Rekom Biotech is committed to ensuring the highest level of quality in the design and manufacture of our IVD reagents. Our products are designed, developed, manufactured, and distributed in accordance with our quality system, which is certified under the ISO 13485 standards. Our IVD reagents are manufactured following Standard Operating Procedures (SOPs) and undergo rigorous quality control in our laboratories. You can consult our system policy if you're interested.
We are authorized to work with genetically modified organisms (GMOs), under license number A/ES/19/I-22, issued by the National Biosafety Commission.
We are registered as an INNOVATIVE SME.
Quality Controls
Each batch undergoes various quality controls:
Since the precise determination of extinction coefficients is direct, ultraviolet absorption spectroscopy is preferred over chemical methods, such as the Lowry or Bradford methods. Protein concentration measurement is performed using the theoretical extinction coefficient of the recombinant protein obtained from Gill and vonHippel, 1989.
However, for proteins that do not contain Trp residues, experience shows that this may result in more than 10% error in the calculated extinction coefficient. Therefore, we measure protein concentration using the colorimetric assay based on the interaction between Coomassie Brilliant Blue and arginine and aromatic residues (Bradford method) with a maximum absorption shift from 470 nm to 595 nm (Bradford, 1976).
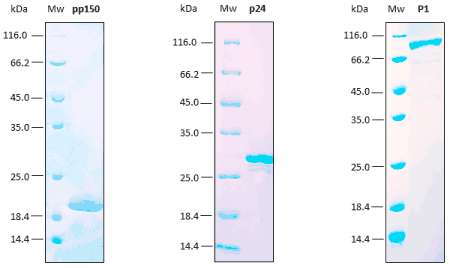
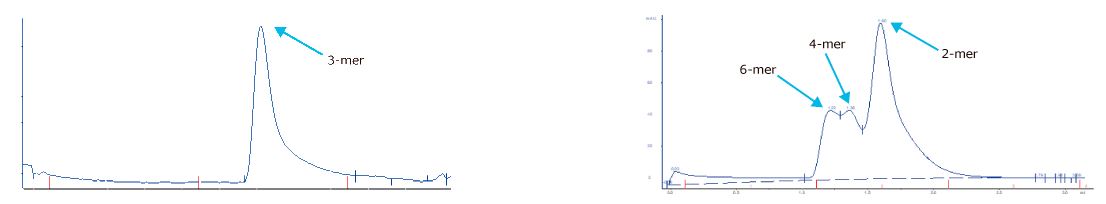
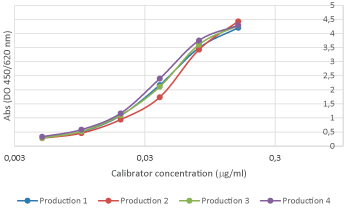
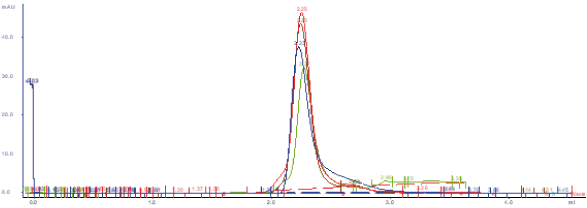
Reproducibility analyses are performed through SDS-PAGE, SEC, and ELISA assays. Excellent replicability of the production process.
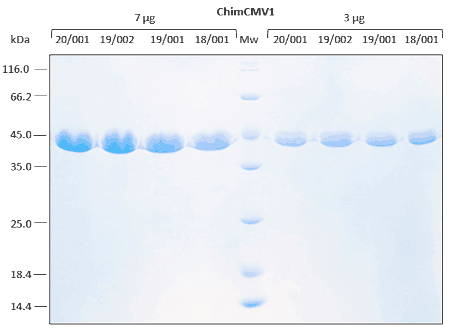
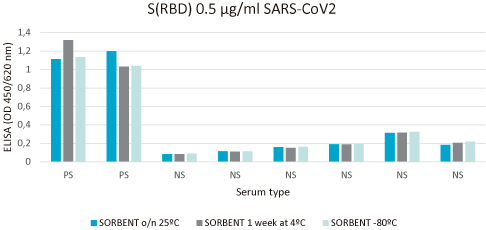
Relative stability is performed with immunoassay analysis under different environmental conditions.
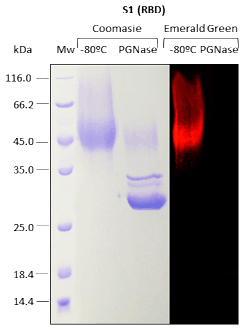
For recombinant proteins produced in Pichia pastoris, N-glycosylation and O-glycosylation are analyzed.
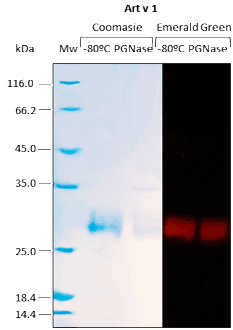
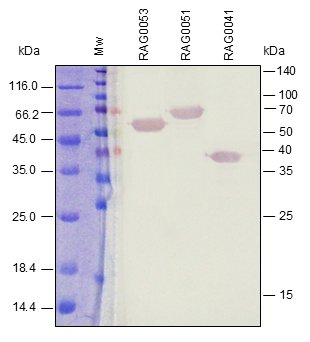
Western BLOT
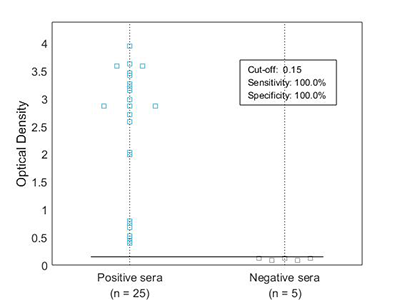
ELISA
Our in vivo monobiotinylated antigens are analyzed using a western blot assay with conjugated streptavidin (A) and several ELISA assays (B), including an indirect ELISA on streptavidin-coated microtiter plates (Figure 1B), and a capture ELISA with the biotinylated recombinant antigen used as a detector with streptavidin-HRP (Figure 2B).
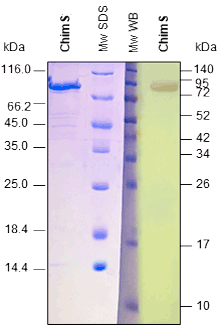
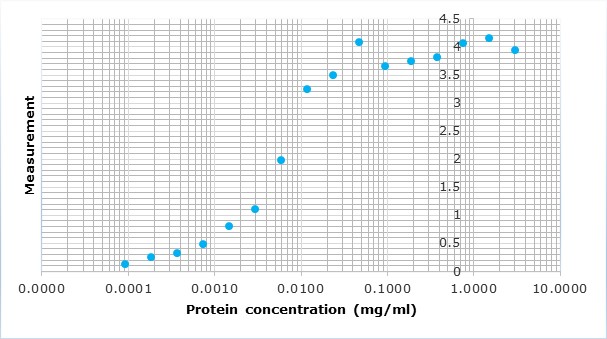
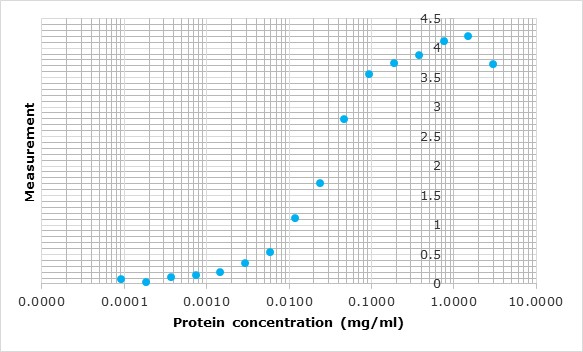
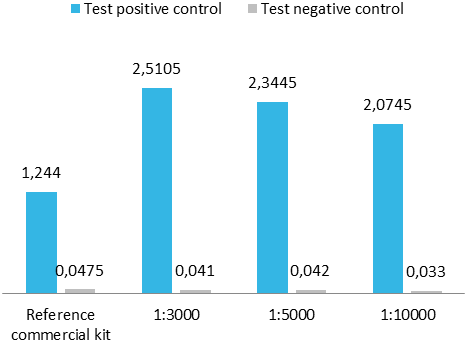
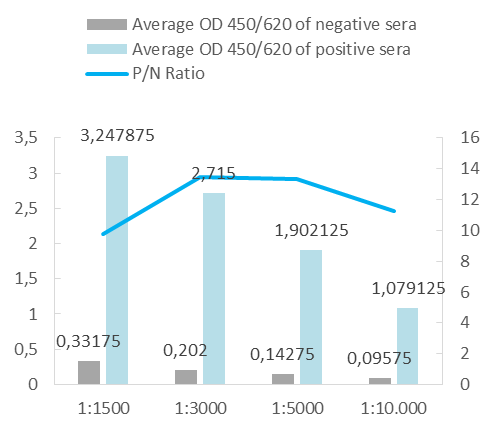
Upon client request or as an internal quality control for a capture ELISA format, we occasionally conjugate our antigens with peroxidase. For analysis, we perform a capture ELISA using a commercial test and an in-house assay.
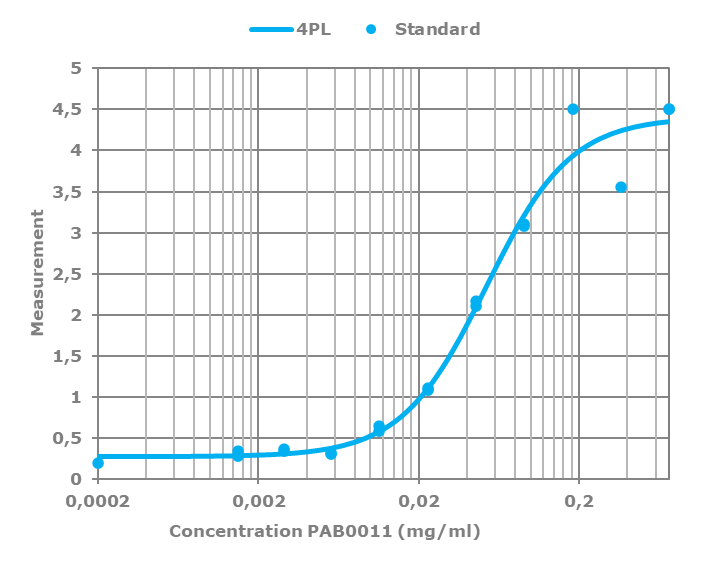
All polyclonal antibodies are titrated against their immunogen by varying the antibody concentration against a fixed amount of immunogen on the plate. The resulting curve is then fitted to a four-parameter logistic regression, or 4PL.
Boost your laboratory with custom reagents
At Rekom Biotech, we design and manufacture high-quality, validated, and versatile IVD reagents for reliable and effective in vitro diagnostics.
-
[[carrito.product.name]]
- [[sku.sku]]
Or if you prefer...
As manufacturers, we can tailor our products to your needs.
-
[[carrito.product.name]]
- [[sku.sku]]
As manufacturers, we can adapt our products to your needs.
Contact us!
