Monobiotinylated recombinant proteins
Service of monobiotinylated proteins with biotin at the C-terminal end, designed to ensure specific binding to streptavidin
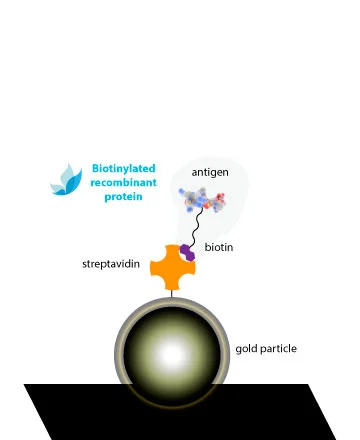
In Rekom Biotech we have developed a product line of monobiotinylated proteins, offering some of our catalog numbers with a biotin in their C-terminus. This molecule allows the specific interaction of biotinylated proteins to streptavidin.
Our biotinylated proteins are bonded to a BCCP-tag in the C-terminus, with a lysine residue which is enzymatically biotinylated by the E. coli biotin ligase BirA. This single-point labelling technique has many advantages for commonly used binding assays:
- The biotinylation only happens on the lysine residue of the BCCP tag.
- There is NO interference with the target protein’s natural binding activities.
- The protein orientation is uniform when immobilized on a streptavidin-coated surface such as nanoparticles.
Catalog of monobiotinylated recombinant proteins
DISEASE
Microorganismo
Tipo de reactivo
- Alérgeno / Antígeno
- Bloqueador
- Anticuerpo
Características Reactivos
- Enfermedad humana
- Enfermedad animal
- Alergia
-
[[ dis.node_type === 'allergie' ? dis.scientificname : '' ]] [[ dis.node_type !== 'allergie' ? dis.microorganism : '' ]] [[ dis.node_type === 'allergie' ? '(' + dis.title + ')' : '' ]]-
[[prod.name]]
- [[sku.sku]] [[sku.subsku]]
-
[[prod.name]]
No results found
Boost your laboratory with customized reagents
At Rekom Biotech, we design and manufacture high-quality, validated, and versatile IVD reagents for reliable and effective in vitro diagnostics.
-
[[carrito.product.name]]
- [[sku.sku]]
Or if you prefer...
Contact us, we can tailor our products to your needs.
-
[[carrito.product.name]]
- [[sku.sku]]
As manufacturers, we can adapt our products to your needs.
Contact us!
Conventional chemical conjugation
A conjugated antigen can be a helpful solution for various issues that can arise during the development of a new IVD test. One common problem caused by most surfaces is the denaturation of proteins due to their high surface hydrophobicity.
Furthermore, the binding events may be affected by the position of the surface and sensor molecules, which could cause a greater steric impact. Moreover, positioning the molecules in a specific orientation could enhance the stability of the attached proteins and make the assay more sensitive by exposing its antigenic regions.
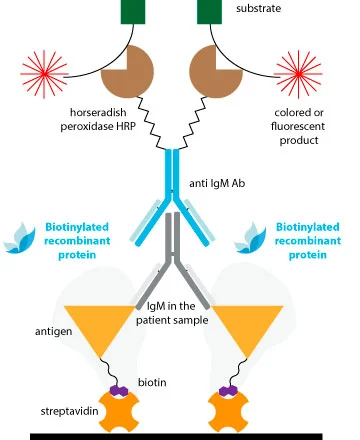
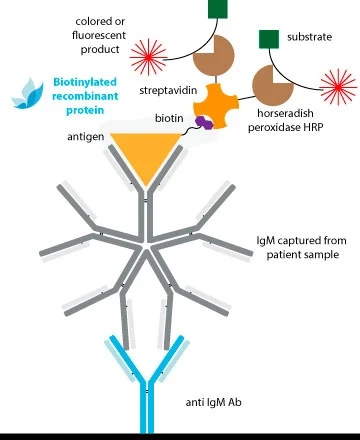
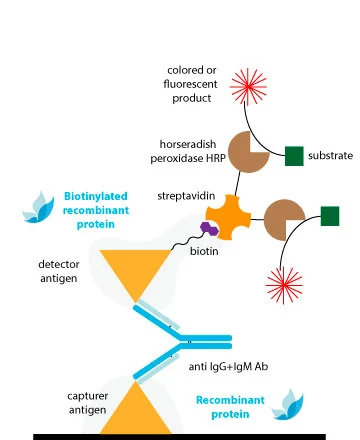
Traditional methods of conjugation are effective for antibodies, but they may not provide consistent results for antigens with less established structures. This may explain why Double-Antigen Sandwich ELISAs (DAgS) are not as widely used for detecting antibodies compared to indirect ELISA.
To avoid compromising the antigenic structure and sensitivity of DAS-ELISAs due to conventional chemical conjugation, the ideal solution is to utilize monobiotinylated proteins.
Why use our monobiotinylated proteins?
The extremely specific and high affinity binding of biotin by avidin and/or streptavidin (Kd ≈ 10-14M) results in specific detection systems of very high sensitivity. A clear advantage of this system is that with a common strep-HRP, we can obtain conjugated complex of all our references without the necessity of performing the peroxidation of each one.
The biotin is fused to a linker which maintains the molecule away to the protein surface, avoiding steric hindrance between the biotin and the antigenic regions involved in Ab-binding. Thus, the Ab interaction will not be compromised with the conjugation.
As there is just one biotin per protein molecule, our conjugated proteins will show a higher inter-lot reproducibility and this will facilitate the reproducibility of the diagnostic tests developed with them.
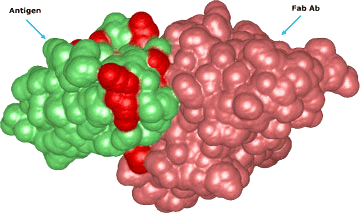
Uses of monobiotinylated proteins